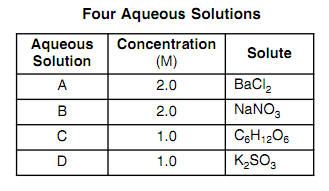
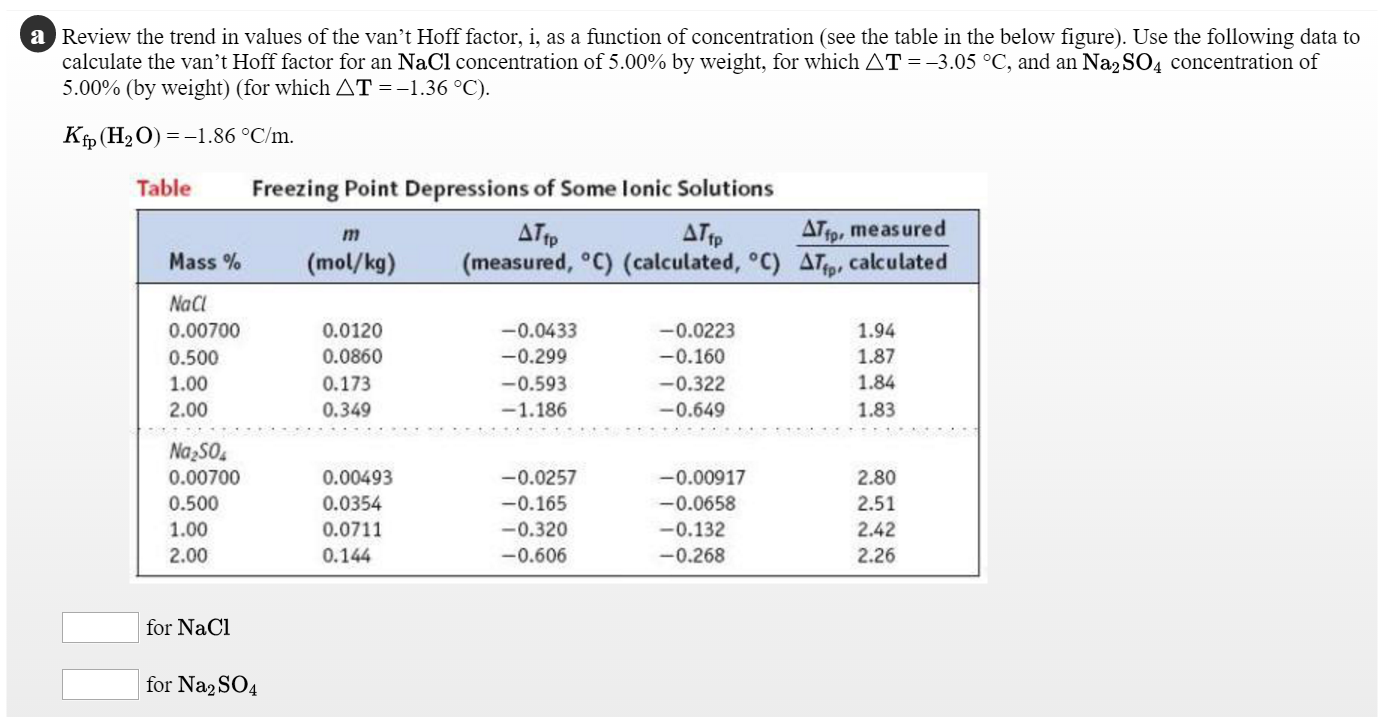
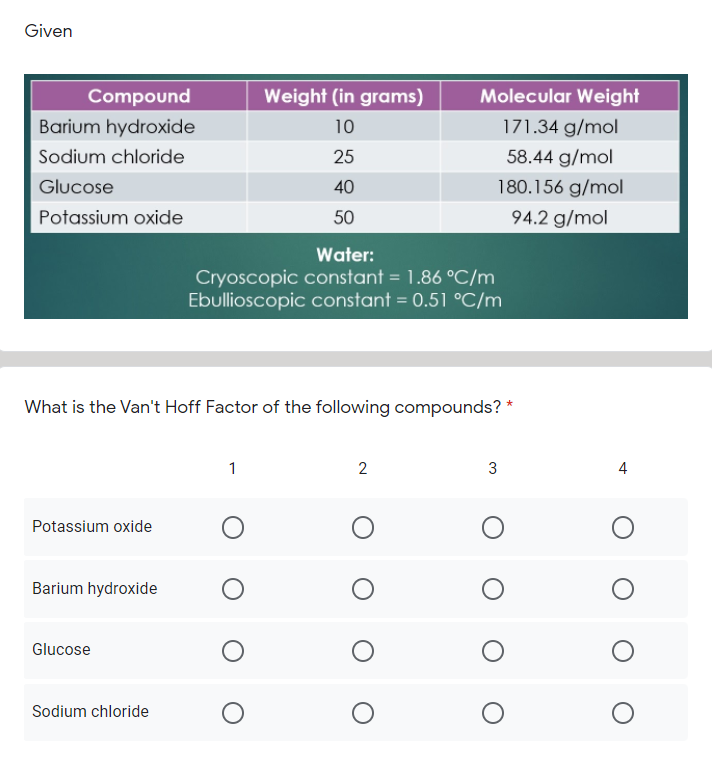
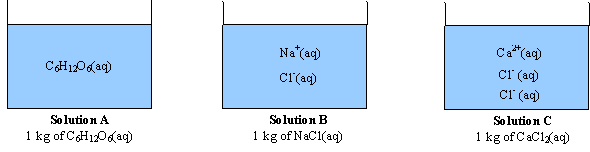Van't Hoff factor as a function of concentration (calculated according... | Download Scientific Diagram

At what temperature would 1.3 m NaCl freeze, given that the van 't Hoff factor for NaCl is 1.9? Kf for water is 1.86 degrees C/m. | Homework.Study.com
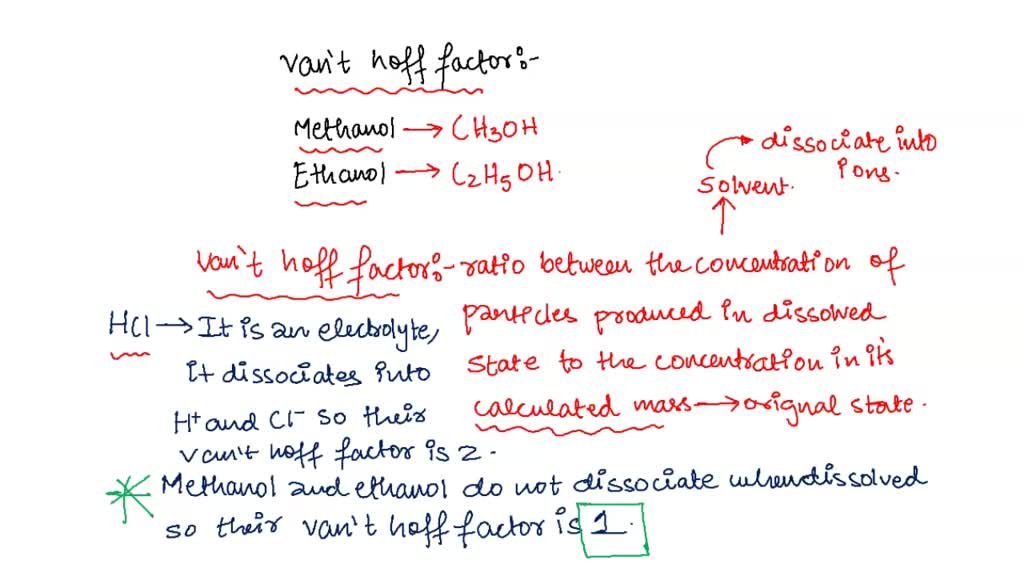
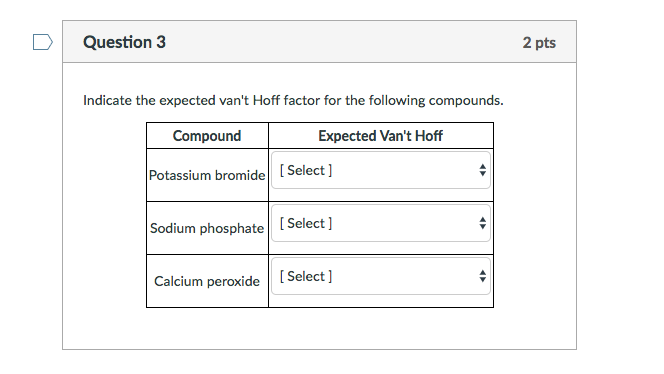
SOLVED: Question 2 0.5 pts What is the van't Hoff factor for methanol? Question 3 0.5 pts What is the van't Hoff factor for ethanol?
What is the van 't Hoff factor for the NaCl in aqueous solution (assuming the complete dissociation of NaCl)? - Quora