Metaphosphoric acid, 39-43%, bal. NaPO{3} (Stabilizer), Thermo Scientific Chemicals, Quantity: 100 g | Fisher Scientific
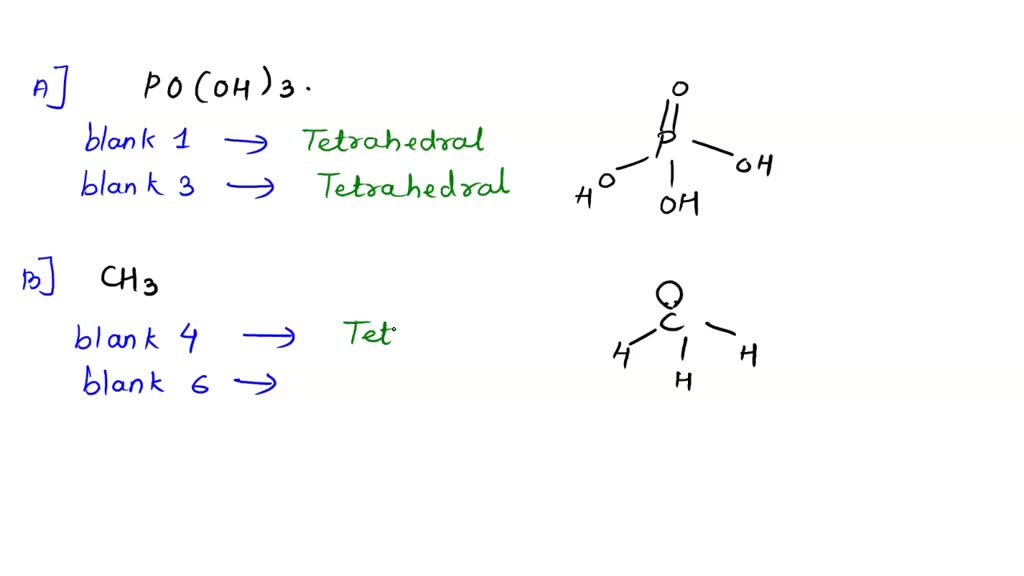
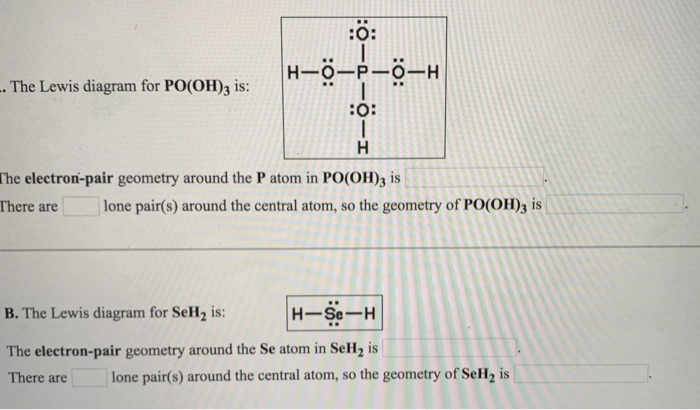
SOLVED: A. The Lewis diagram for PO(OH)3 is: The electron-pair geometry around the P atom in PO(OH)3 is fill in the blank 1. There are lone pair(s) around the central atom, so
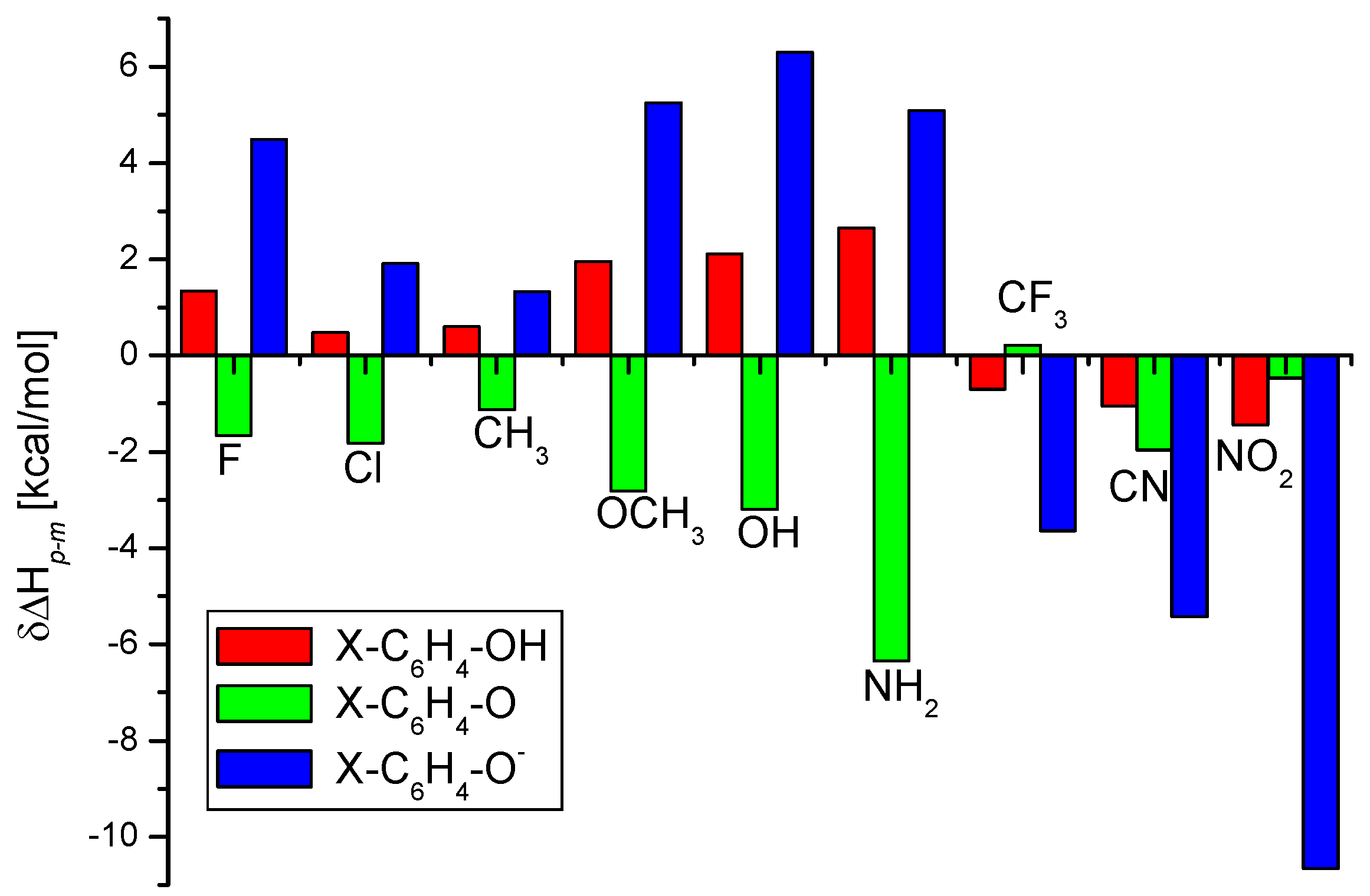
IJMS | Free Full-Text | The O-H Bond Dissociation Energies of Substituted Phenols and Proton Affinities of Substituted Phenoxide Ions: A DFT Study

Why is phosphorous acid H3PO3 and not P(OH)3 - which should be more accurate as per the molecule structure? - Quora
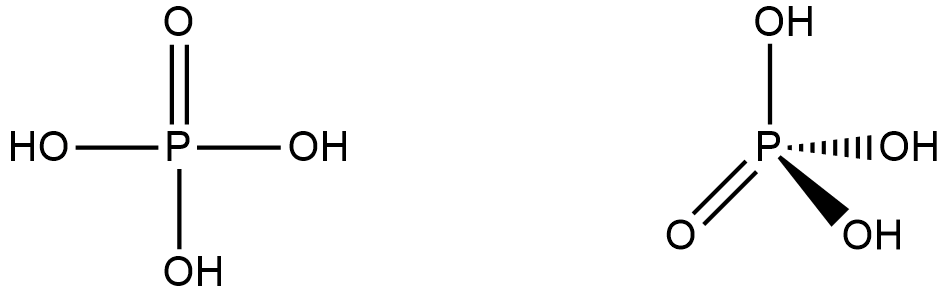
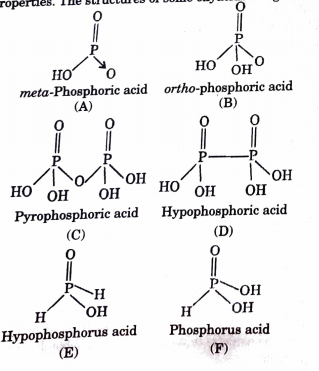
How many P = O bonds and P – OH bonds (respectively) are present in orthophosphoric acid?A.2,1B.3,3C.1,3D.4,3
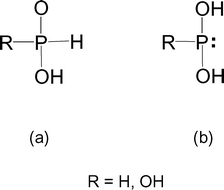
Stabilization of the tautomers HP(OH) 2 and P(OH) 3 of hypophosphorous and phosphorous acids as ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/B510479C

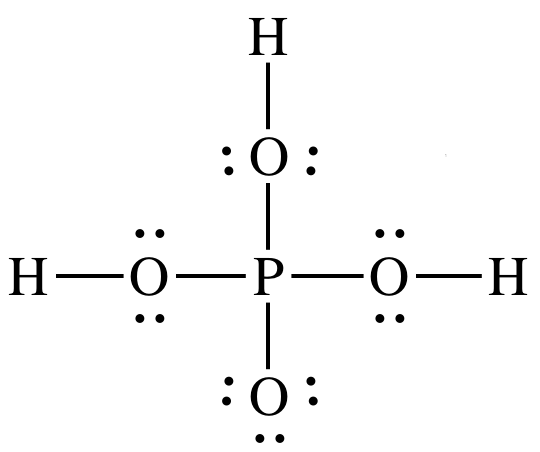
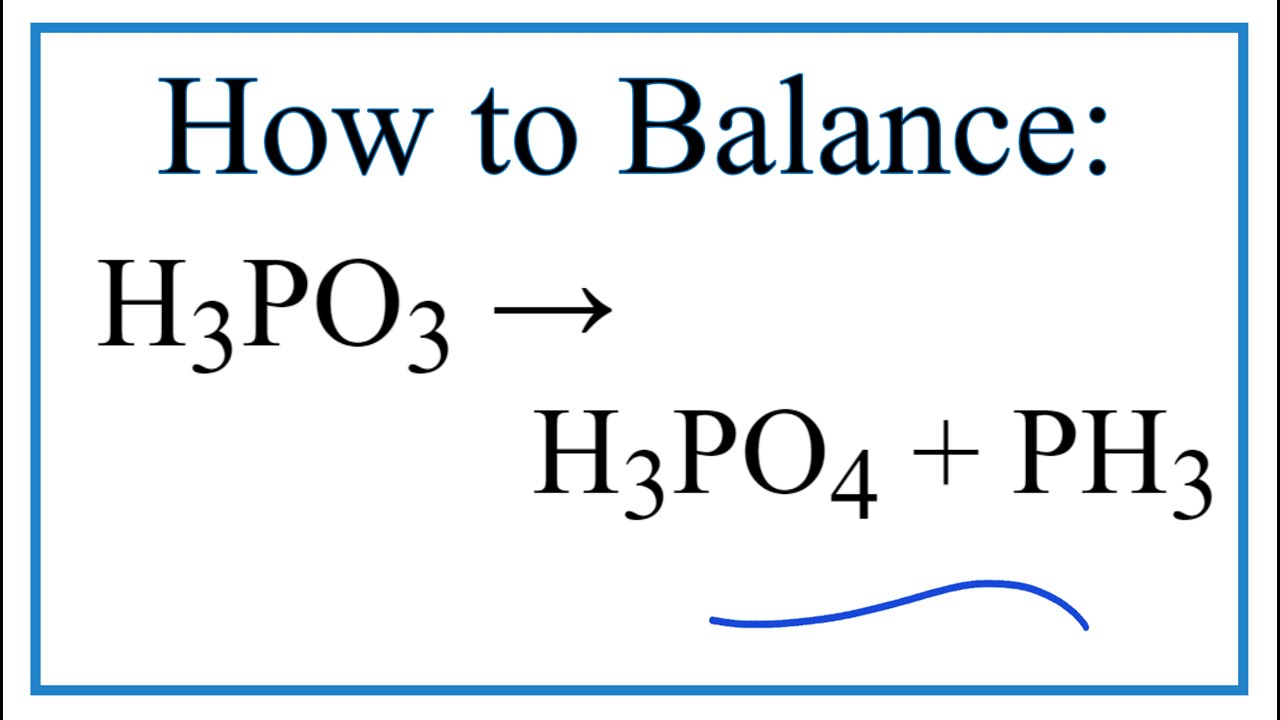
Phosphorous acid, H_3PO_3, has the structure (HO)_2PHO, in which one H atom is bonded to the P atom, and two H atoms are bonded to O atoms. For each bond to an


/chapter6/pages21and22/page21and22_files/pbr3mechanism.png)



![Calculating pH, pOH, [H+] and [OH-] of Common Substances | TPT Calculating pH, pOH, [H+] and [OH-] of Common Substances | TPT](https://ecdn.teacherspayteachers.com/thumbitem/Calculating-pH-pOH-H-and-OH-of-Common-Substances-3600412-1657170541/original-3600412-3.jpg)