
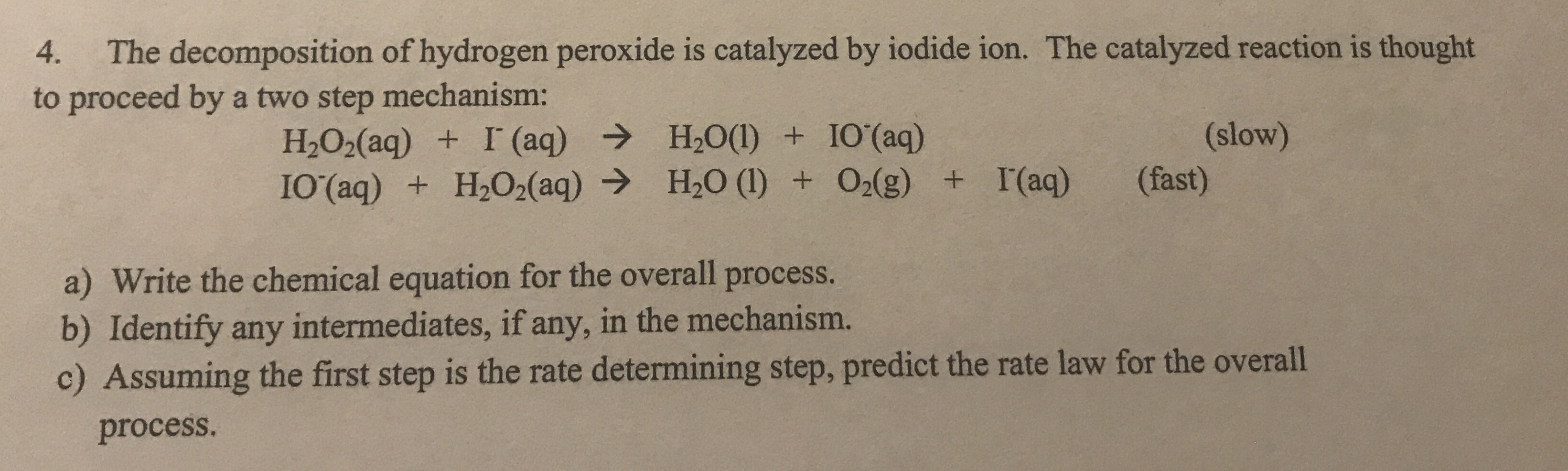
H2O2 is decomposed to H2O and O , in the following sequence of reactions :i) H2O2(aq) + I^-(aq)→ H2O(l) + IO^ - (aq) ii) H2O2(aq) + I^-(aq)→ H2O(l) + O2(g) + IO^ - (
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community

Selective H2O2 production on N-doped porous carbon from direct carbonization of metal organic frameworks for electro-Fenton mineralization of antibiotics - ScienceDirect

Peracetic Acid Oxidation of Saline Waters in the Absence and Presence of H2O2: Secondary Oxidant and Disinfection Byproduct Formation | Environmental Science & Technology
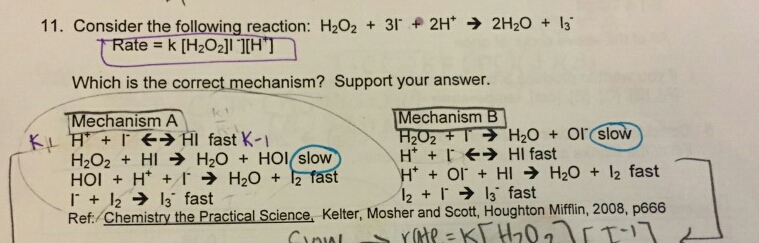
![OneClass: 1. The rate law for the reaction H2O2 + 2H+ + 2I - ? I2 + 2H2Oisrate = k[H2O2][I-]. The fol... OneClass: 1. The rate law for the reaction H2O2 + 2H+ + 2I - ? I2 + 2H2Oisrate = k[H2O2][I-]. The fol...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/97/9772448.png)
OneClass: 1. The rate law for the reaction H2O2 + 2H+ + 2I - ? I2 + 2H2Oisrate = k[H2O2][I-]. The fol...
What is the balanced half-reaction equation for H2O2 (aq) acting as a reducing agent in an acidic aqueous solution? - Quora
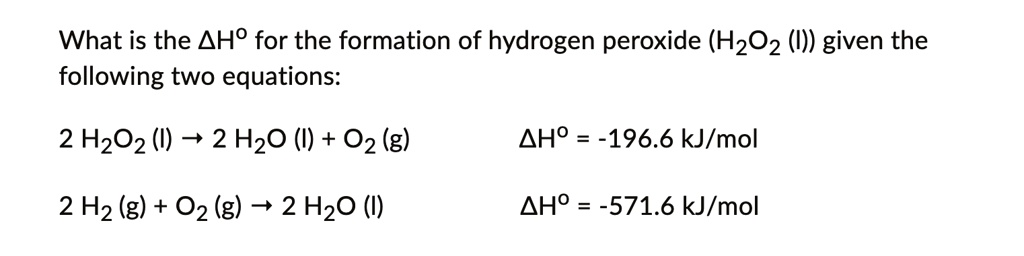
SOLVED: What is the AHO for the formation of hydrogen peroxide (H2O2 (I)) given the following two equations: 2 H2O2 () 2 HzO () +02 (g) AHo -196.6 kJ/mol 2 Hz (g) + 02 (g) 2 Hz0 () AHO = -571.6 kJ/mol

Scheme 5. (i) H2O2, NaOH, THF, t-BuOH, 40% yield; (ii) NH2NH2H2O, HOAc,... | Download Scientific Diagram
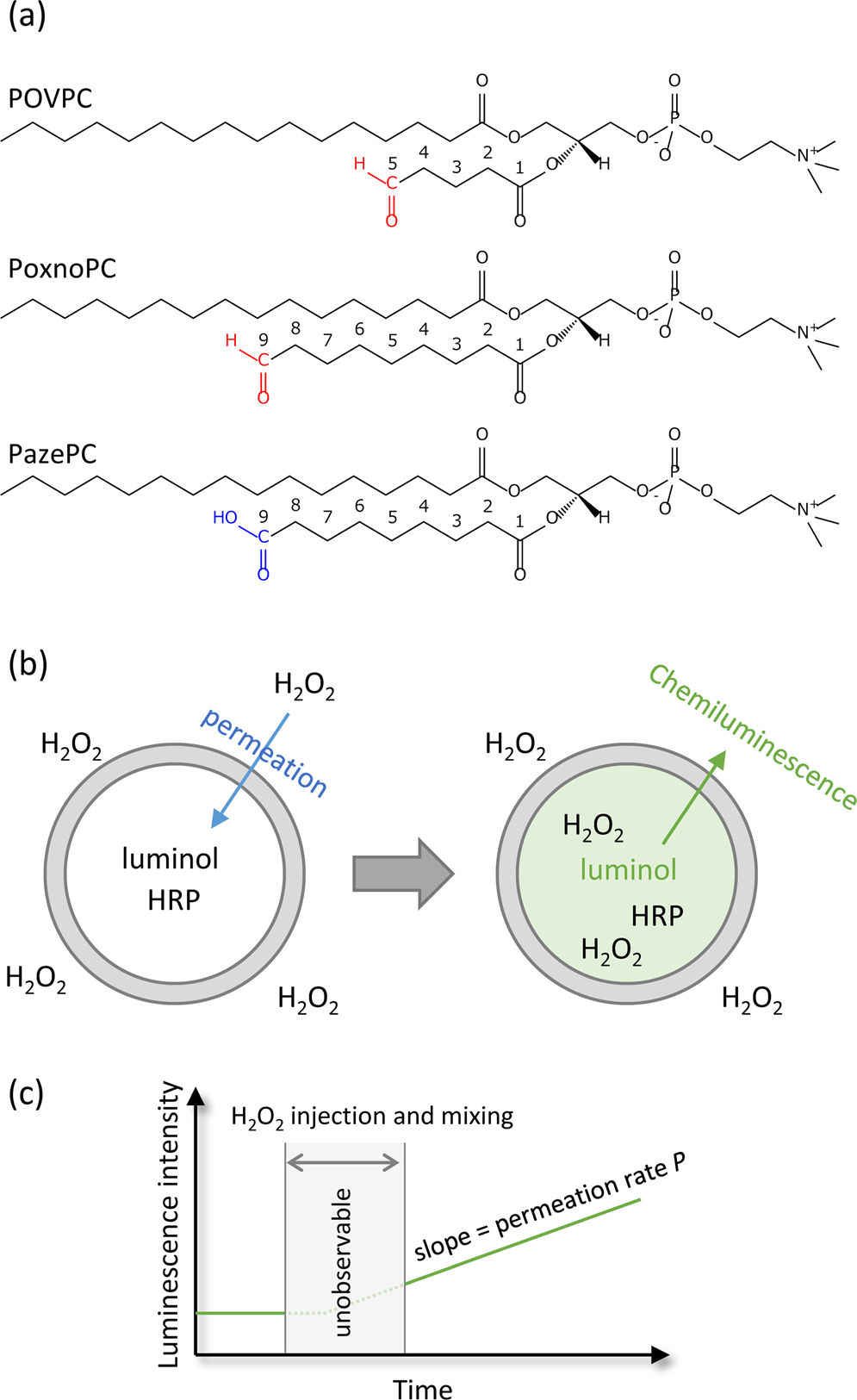
Role of Oxidized Lipids in Permeation of H2O2 Through a Lipid Membrane: Molecular Mechanism of an Inhibitor to Promoter Switch | Scientific Reports








![Hydrogen Peroxide [H2O2] Molecular Weight Calculation - Laboratory Notes Hydrogen Peroxide [H2O2] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/hydrogen-peroxide-molecular-weight-calculation-300x196.jpg)


![Integrated rate laws ln[A] = -kt + ln[A]0 rate = k[A] - ppt download Integrated rate laws ln[A] = -kt + ln[A]0 rate = k[A] - ppt download](https://slideplayer.com/slide/14728131/90/images/4/Reaction+mechanism+2H2O2+%28aq%29+%EF%82%AE+2H2O%28l%29+%2B+O2%28g%29+rate+%3D+k%5BH2O2%5D+%5BI-%5D.jpg)