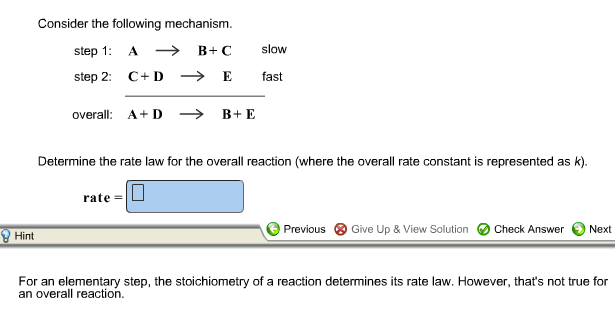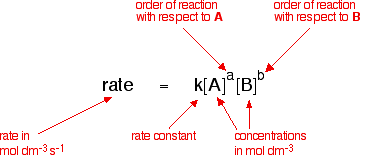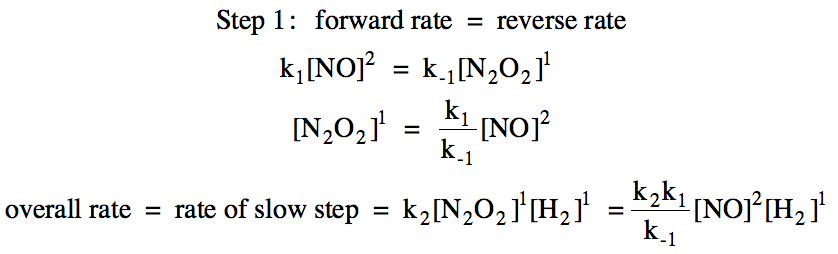
Determine the rate law for the overall reaction (where the overall rate constant is represented as k) - Home Work Help - Learn CBSE Forum

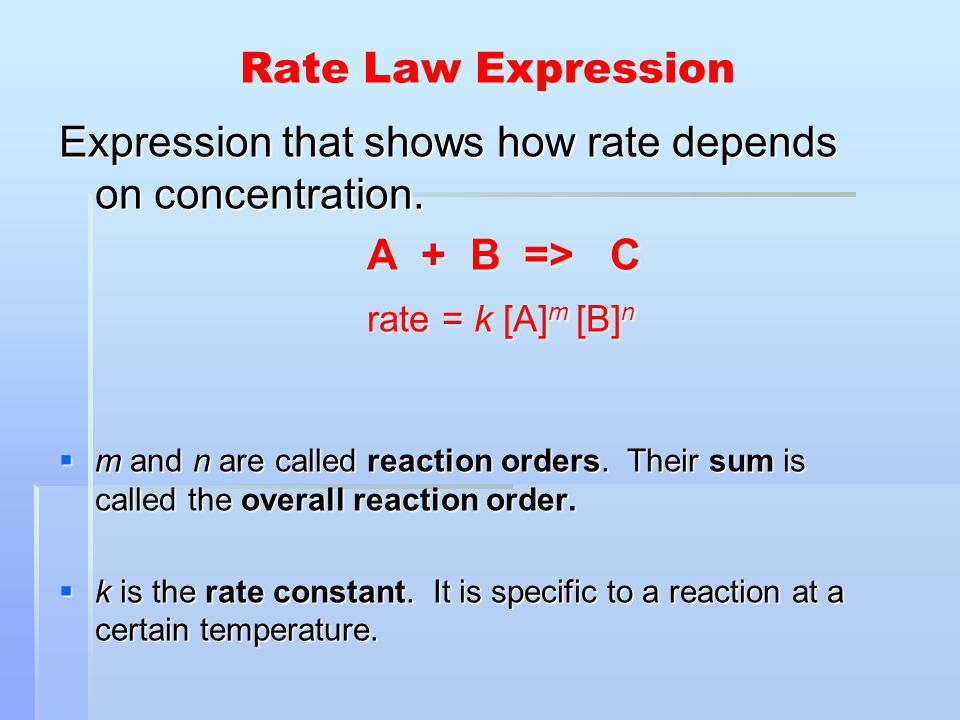
The Rate Law. Objectives: To understand what a rate law is To determine the overall reaction order from a rate law CLE ppt download

A reaction takes place in three steps. The rate constant of the three steps is K1 , K2 and K3 respectively. The overall rate constant K = K1K3/K2 . The energy of

How to Determine the Order of Reaction by Comparing Initial Rates of Reactions | Chemistry | Study.com

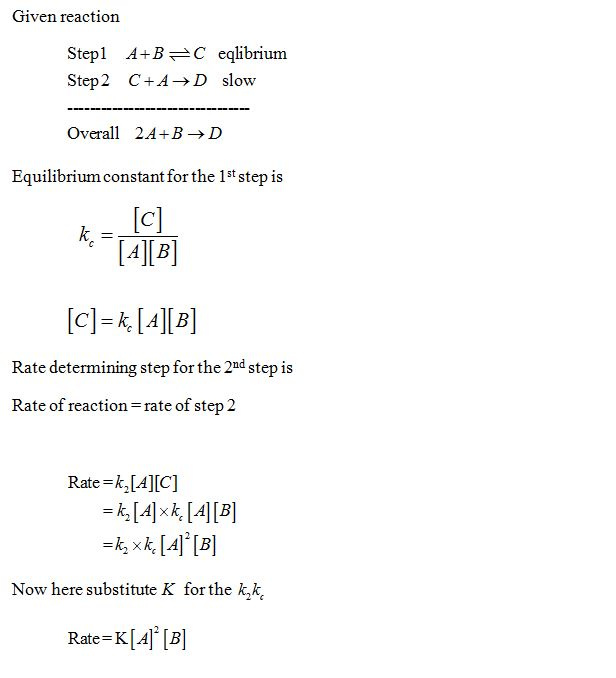
SOLVED: Consider the following mechanism step 1: 2A B+ C equilibrium step 2: B+ D 57 E slow overall: 2A+ D C+ E Determine the rate law for the overall reaction (where
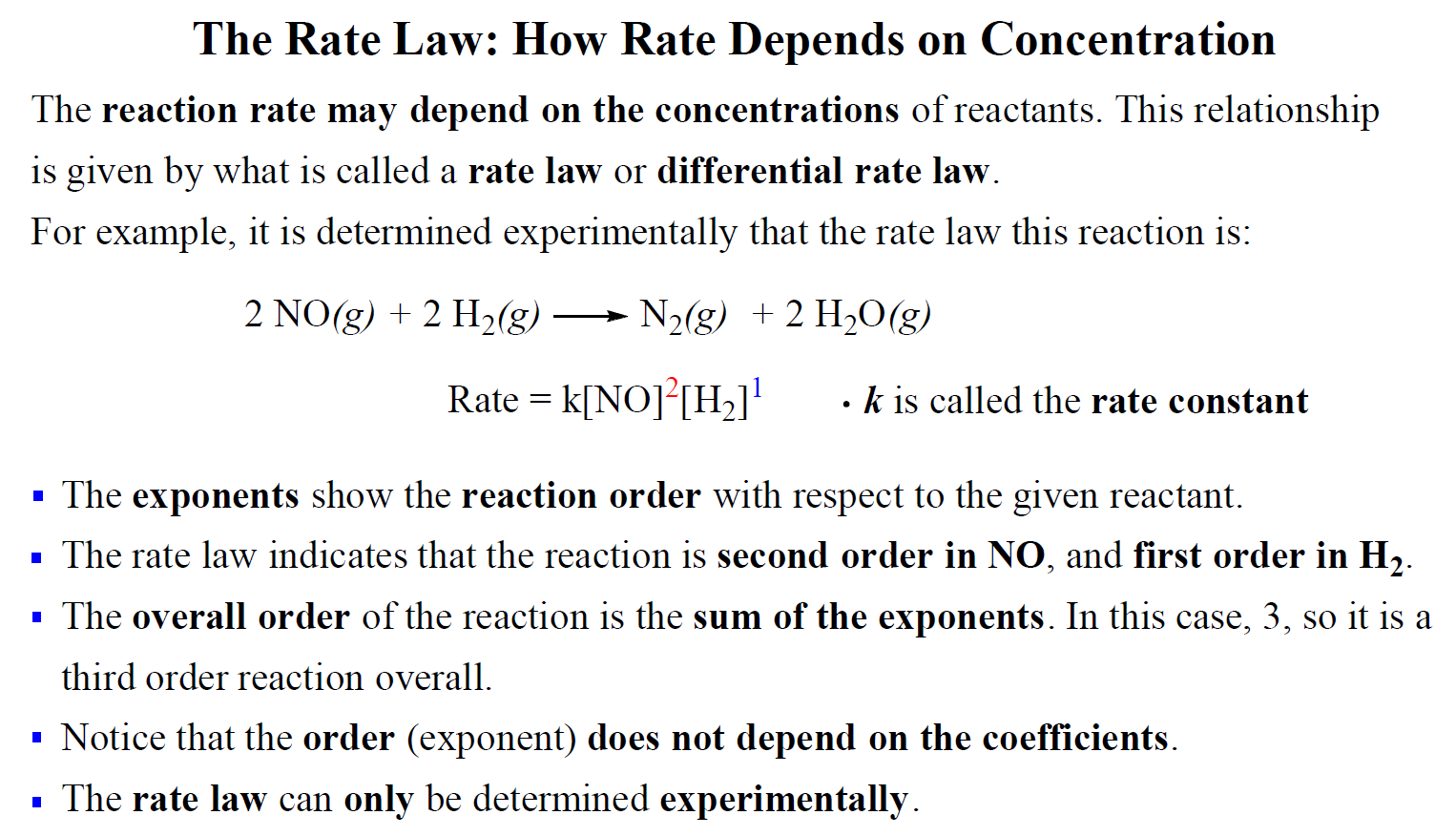
![I know the overall rate law is r=k[NO]^2[H2]^1, and that the RDS has the rate law in it with its coefficients, but there are 1) no speeds and 2) there are 2 I know the overall rate law is r=k[NO]^2[H2]^1, and that the RDS has the rate law in it with its coefficients, but there are 1) no speeds and 2) there are 2](https://preview.redd.it/i-know-the-overall-rate-law-is-r-k-no-2-h2-1-and-that-the-v0-we51rd60enda1.png?auto=webp&s=4ee8efdae71dba86eac26ee1ffe459617a39f65c)
I know the overall rate law is r=k[NO]^2[H2]^1, and that the RDS has the rate law in it with its coefficients, but there are 1) no speeds and 2) there are 2
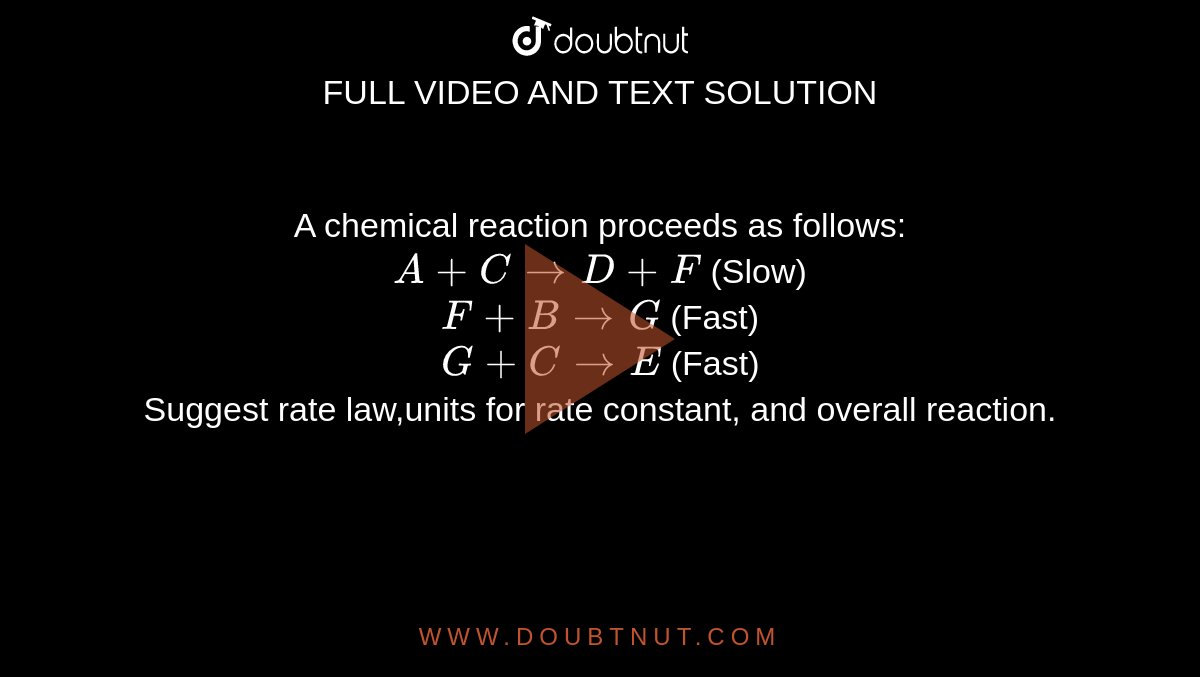
A chemical reaction proceeds as follows: A + C rarr D + F (Slow) F + B rarr G (Fast) G + C rarr E (Fast) Suggest rate law,units for rate constant,

Rate Constant Measurements for the Overall Reaction of OH + 1-Butanol → Products from 900 to 1200 K | The Journal of Physical Chemistry A