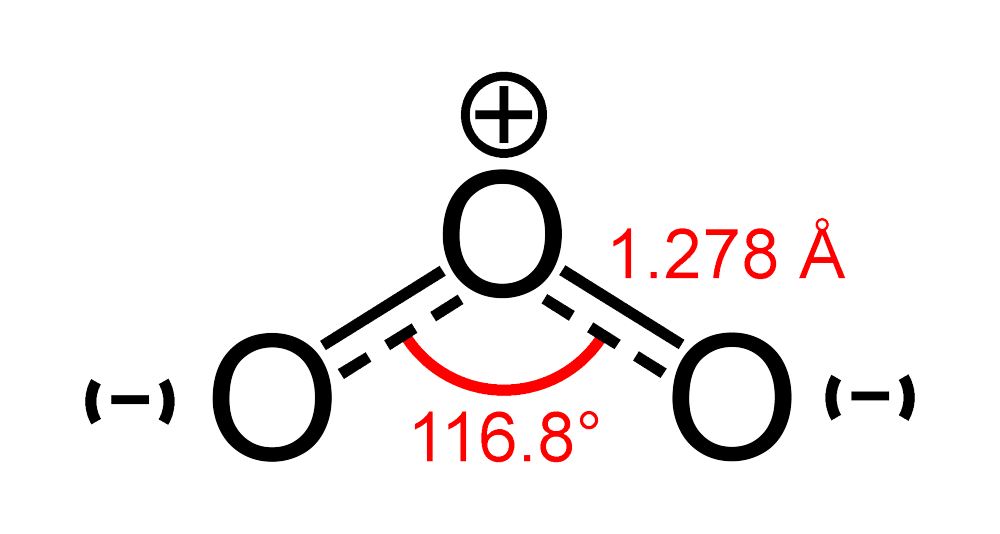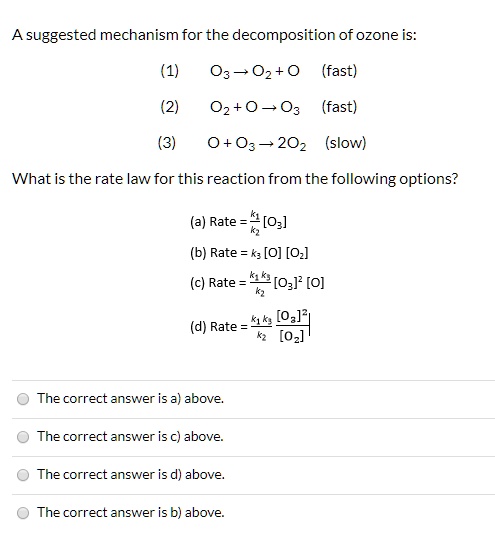
SOLVED: A suggested mechanism for the decomposition of ozone is: O3 0z + 0 (fast) 02 + 0 O3 (fast) 0+O3 202 (slow) What is the rate law for this reaction from

Amazon.com: Enerzen O-922D - Digital Ozone Generator for Eliminating Odors - O3 Machine Air Ionizer Deodorizer with Adjustable Settings for Any Room Size : Home & Kitchen

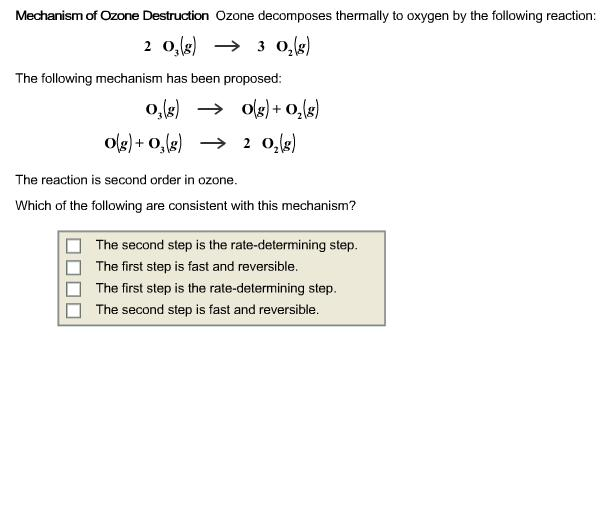
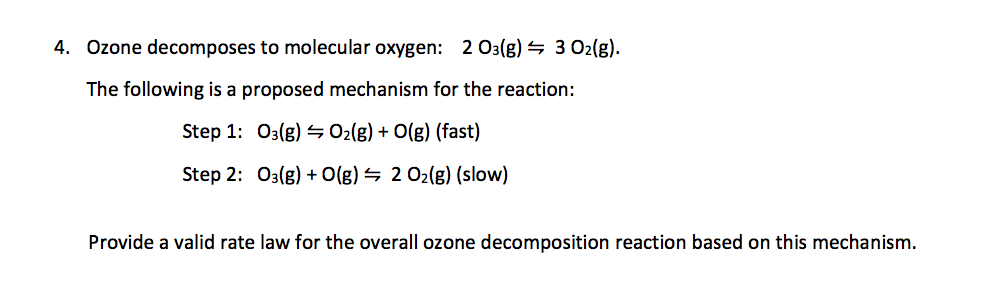
Self-test 22.8 Derive the rate law for the decomposition of ozone in the reaction 2O3(g) → 3O2(g) on the basis of the following mechanism O3 → O2 + O. - ppt video

Formation of O3 in the troposphere. NO2 is cleaved by sunlight to NO• +... | Download Scientific Diagram

On Rates and Mechanisms of OH and O3 Reactions with Isoprene-Derived Hydroxy Nitrates | The Journal of Physical Chemistry A

Amazon.com: Enerzen O-UVC3 - HEPA + UV Light + 40,000 mg/h Industrial Ozone Generator for Eliminating Odors - O3 Machine Air Ionizer Deodorizer for Home, Room, Smoke, Car, Pet : Home & Kitchen
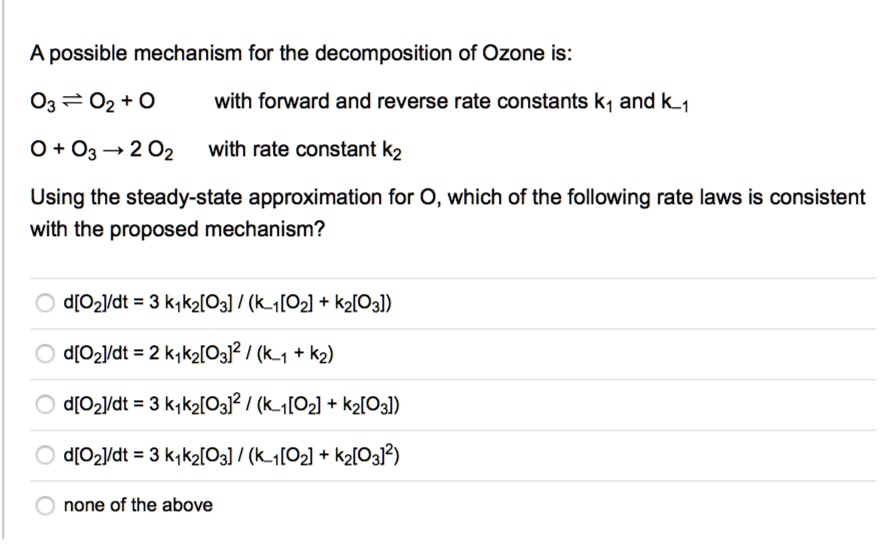
SOLVED: A possible mechanism for the decomposition of Ozone is: O3 = 02 + 0 with forward and reverse rate constants k1 and k1 0 + O3 + 2 02 with rate
![The chemical reaction, 2O2⟶3O2 proceeds as: O3 O2 + [O] (fast) + O3⟶2O2 (slow) The rate law expression will be: The chemical reaction, 2O2⟶3O2 proceeds as: O3 O2 + [O] (fast) + O3⟶2O2 (slow) The rate law expression will be:](https://dwes9vv9u0550.cloudfront.net/images/6173668/3a9b6913-3fa9-46cd-9c10-14063b8916de.jpg)

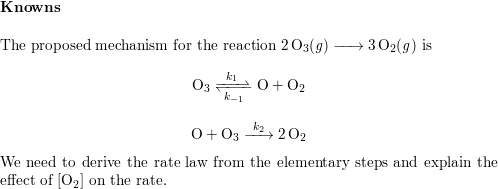



![The [O III]λ5007 EW versus the O3/O2 ratio measured from the VIRUS... | Download Scientific Diagram The [O III]λ5007 EW versus the O3/O2 ratio measured from the VIRUS... | Download Scientific Diagram](https://www.researchgate.net/publication/351448886/figure/fig3/AS:1021754944270337@1620616770835/The-O-IIIl5007-EW-versus-the-O3-O2-ratio-measured-from-the-VIRUS-spectra-for-our.png)