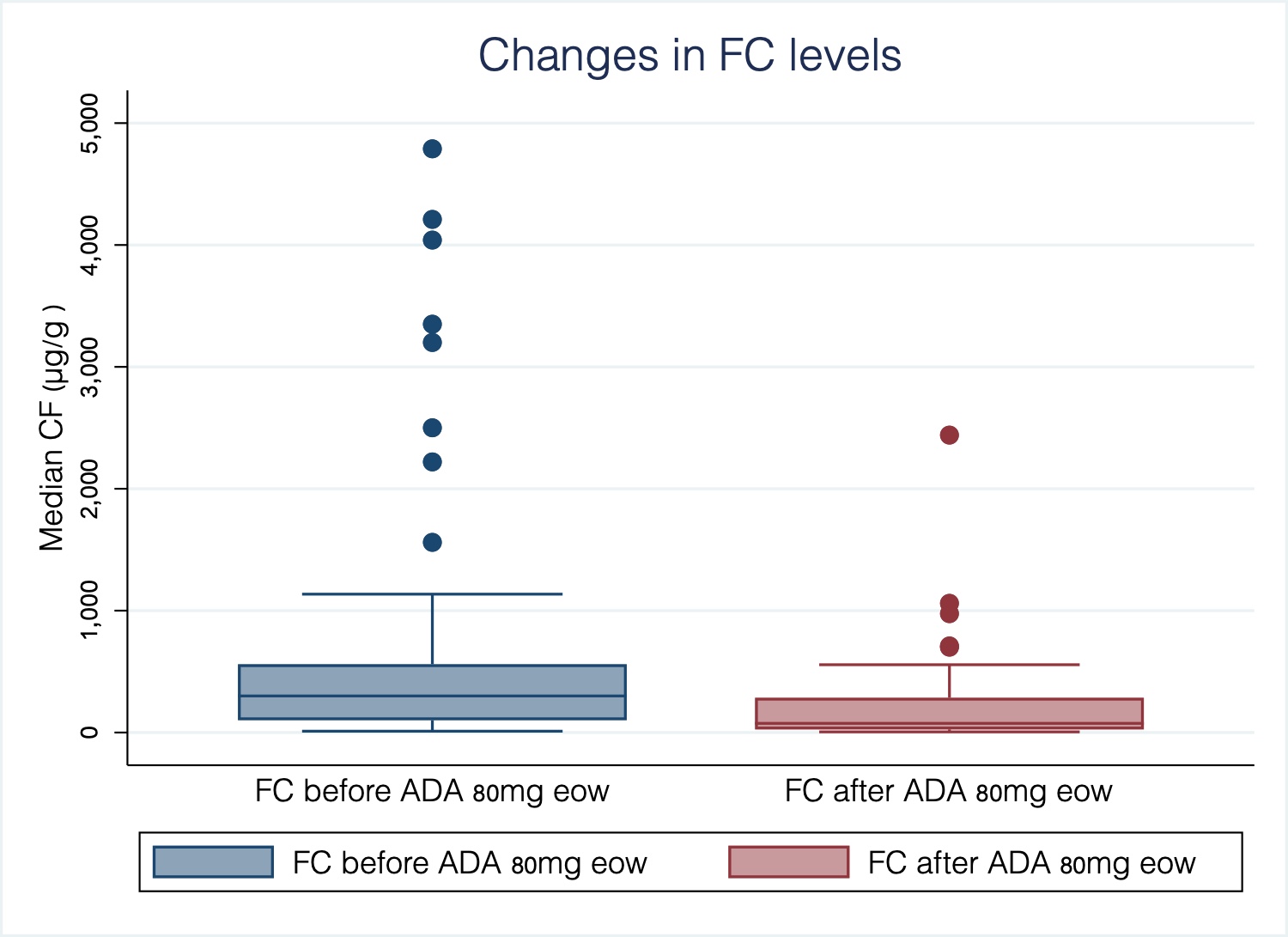
European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice

European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice
![PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/434bc5078bbc62a1a4782f27592fcf28f239679e/8-Table2-1.png)
PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar

Dynamics of circulating TNF during adalimumab treatment using a drug-tolerant TNF assay | Science Translational Medicine
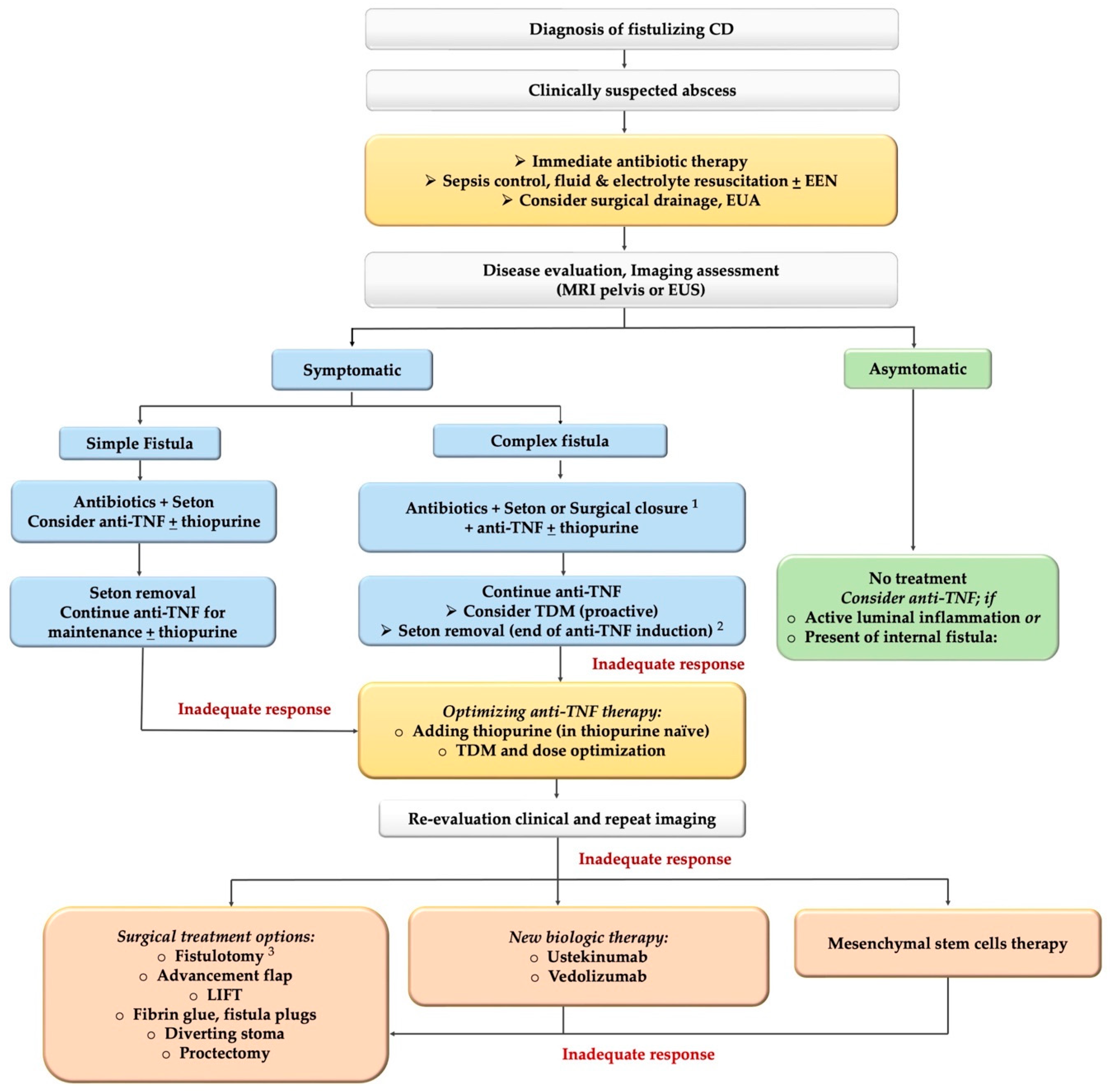
JCM | Free Full-Text | The Optimal Management of Fistulizing Crohn’s Disease: Evidence beyond Randomized Clinical Trials
PDF) Biologics recommendations in the ECCO guidelines on therapeutics in Crohn's disease: Medical treatment
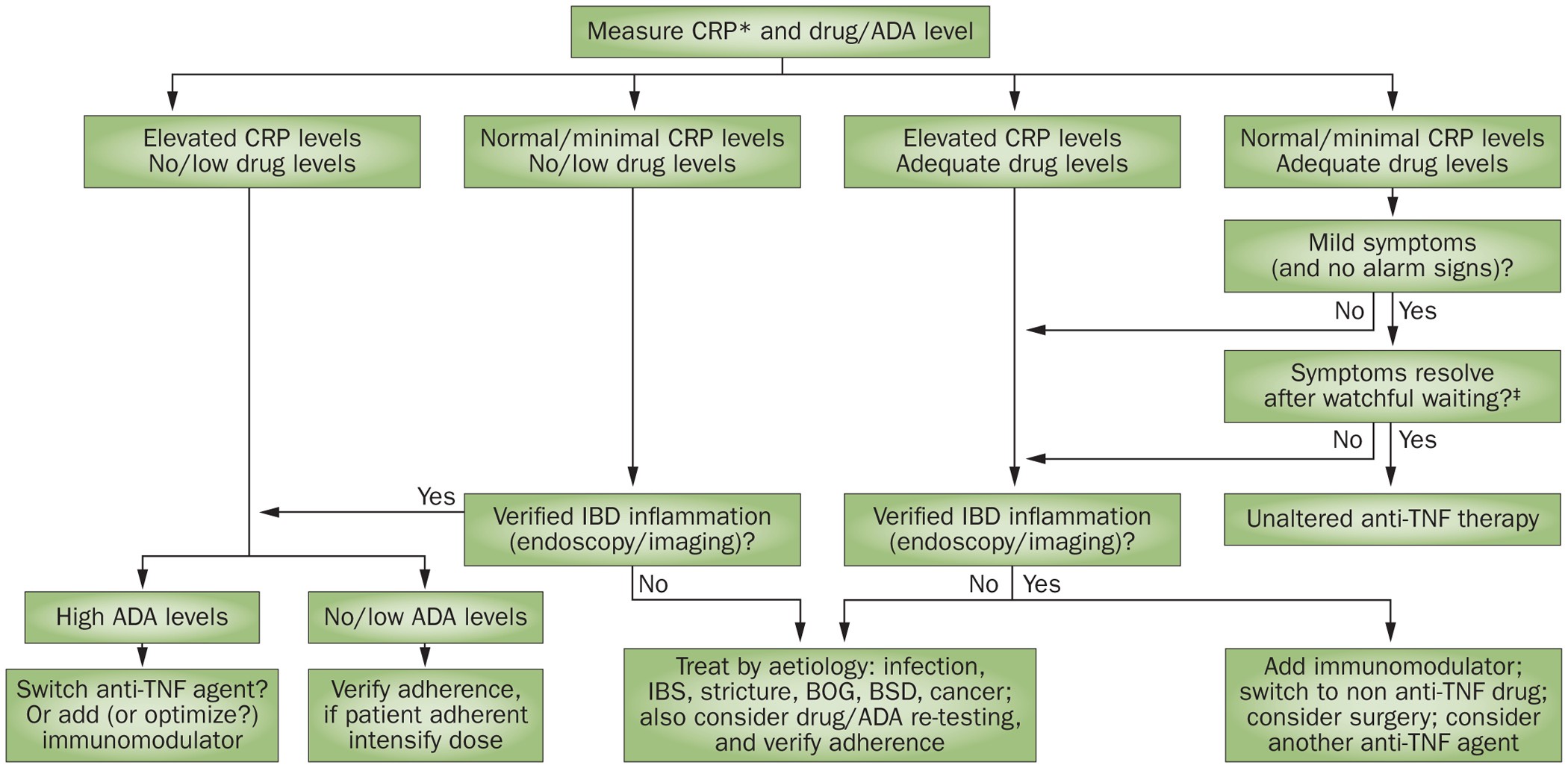
Tailoring anti-TNF therapy in IBD: drug levels and disease activity | Nature Reviews Gastroenterology & Hepatology
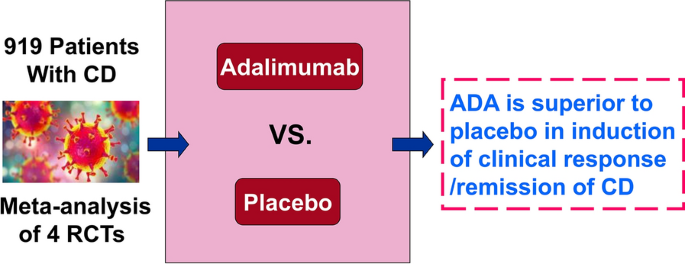
Adalimumab for induction of remission in patients with Crohn's disease: a systematic review and meta-analysis | European Journal of Medical Research | Full Text

Increased versus conventional adalimumab dose interval for patients with Crohn's disease in stable remission (LADI): a pragmatic, open-label, non-inferiority, randomised controlled trial - The Lancet Gastroenterology & Hepatology

Rational Combination Therapy to Overcome the Plateau of Drug Efficacy in Inflammatory Bowel Disease - Gastroenterology
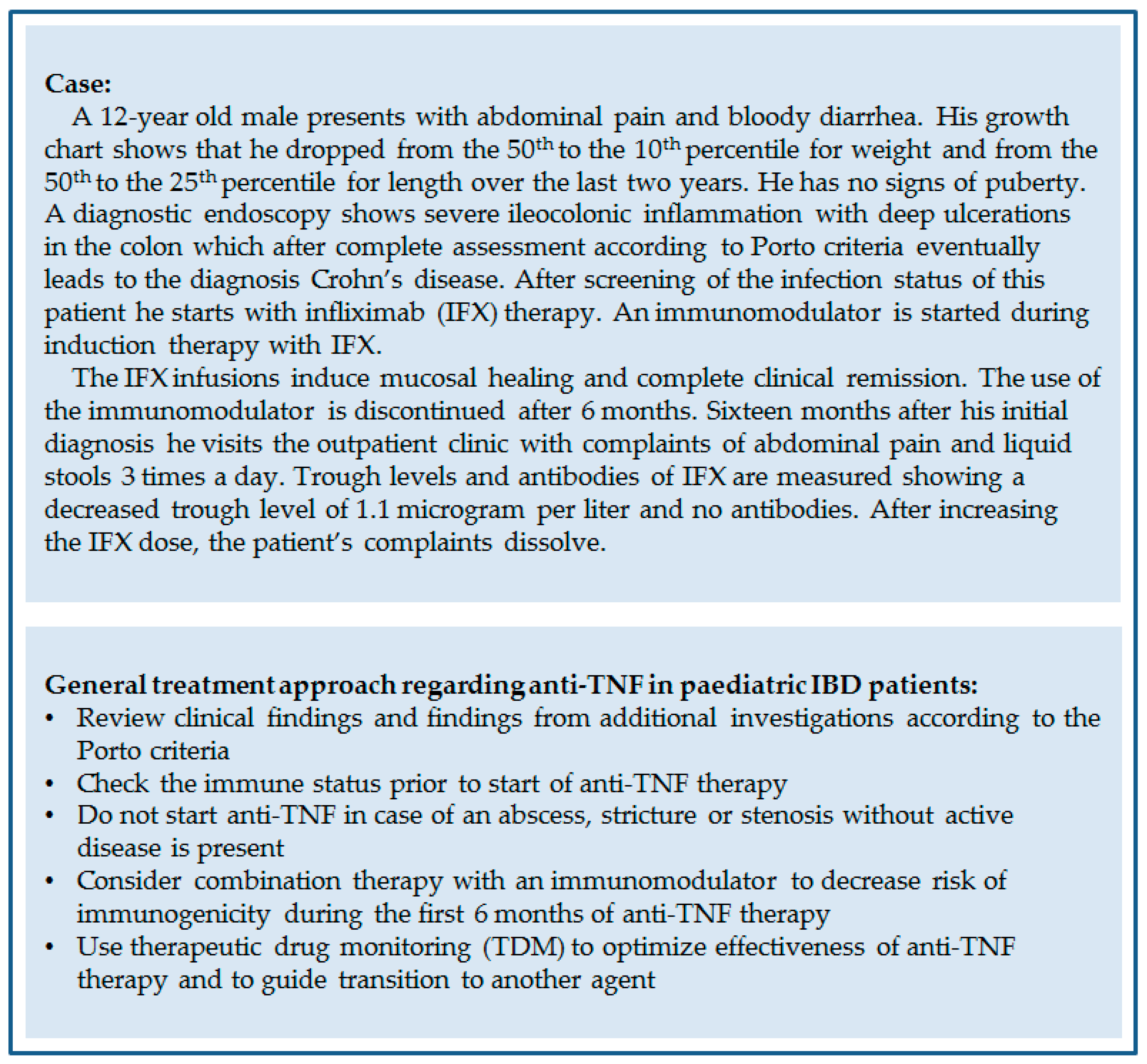
IJMS | Free Full-Text | A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease
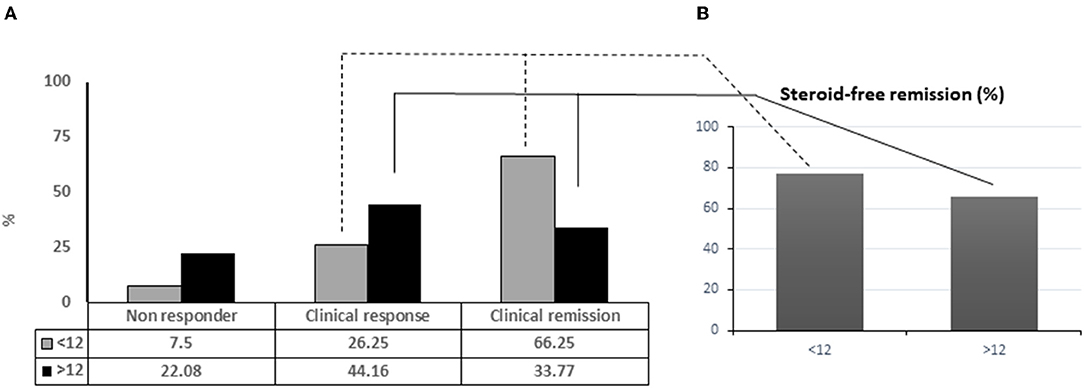
Frontiers | Administration Timing Is the Best Clinical Outcome Predictor for Adalimumab Administration in Crohn's Disease

Children | Free Full-Text | Therapy Strategies for Children Suffering from Inflammatory Bowel Disease (IBD)—A Narrative Review

Immunomodulator Withdrawal From Anti-TNF Therapy Is Not Associated With Loss of Response in Inflammatory Bowel Disease - Clinical Gastroenterology and Hepatology
![PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/434bc5078bbc62a1a4782f27592fcf28f239679e/8-Table1-1.png)
PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar
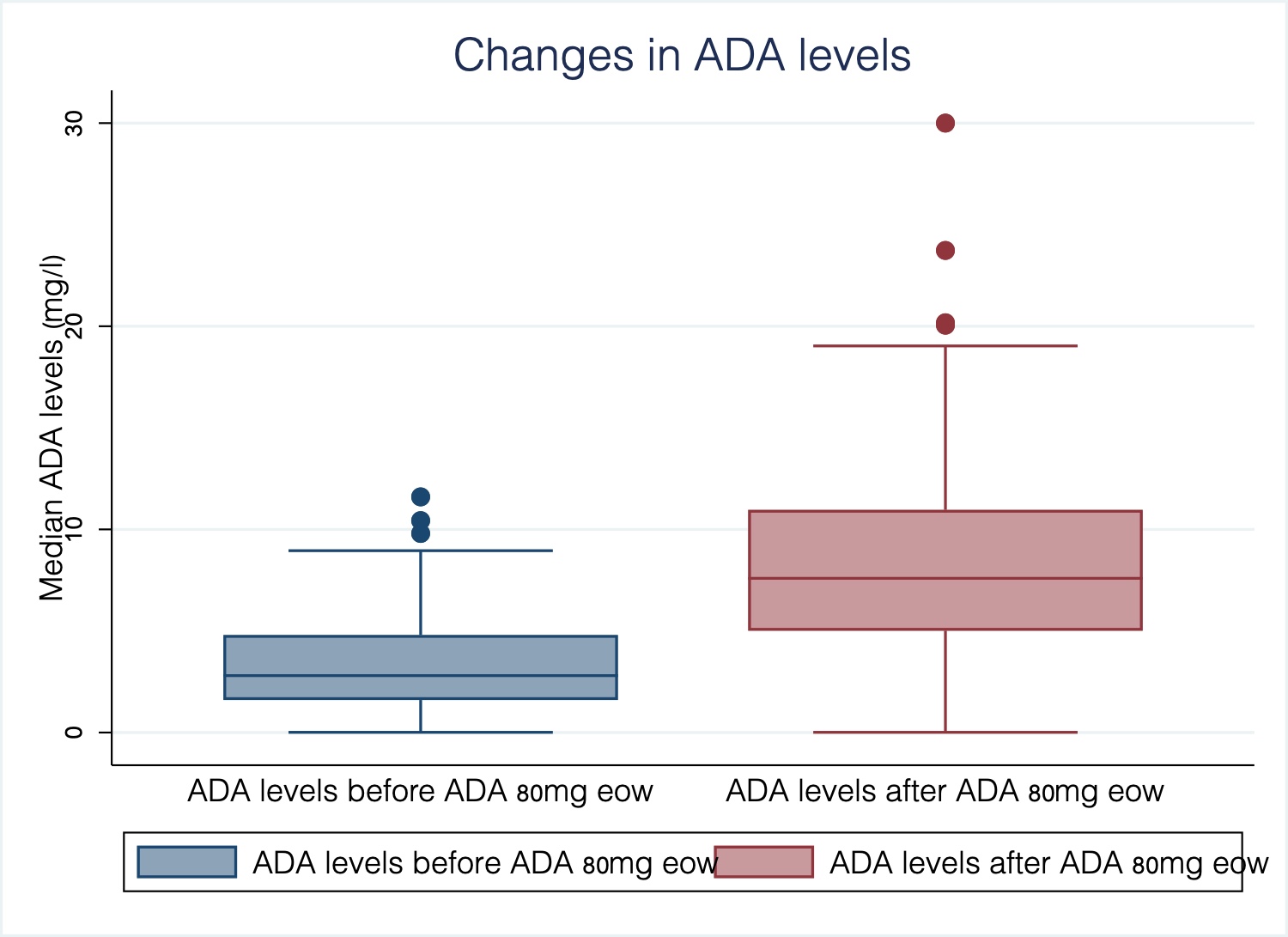
European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice
First-line therapy in adult Crohn's disease: who should receive anti-TNF agents? | Nature Reviews Gastroenterology & Hepatology

PDF) Adalimumab Monotherapy and a Combination with Azathioprine for Crohn's Disease: A Prospective, Randomized Trial

Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator | Scientific Reports